As you know, Aquaponics is based on the nitrogen cycle. Fish produce waste which is basically Ammonia, Bacteria convert them to nitrates that are absorbed by plants.
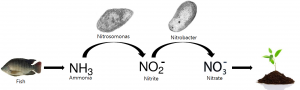
Fish provide ammonia to your system through their waste and from their gills. It is an important player in an aquaponic system. It starts the nitrogen cycle in the system.
Table of Contents
Ammonia in Aquaponics
This chemical with a chemical formula NH3 is an important compound that determines the balance and survival of your aquaponic system. It is produced from fish excretion, uneaten feed, and decomposing organic materials.
Ammonia Poisoning in fish leads to harmful effects like
- Weakened resistance to disease
- Impaired growth
- Damage to tissues, especially in kidneys and gills.
- Physiological imbalance
- Death
Therefore, keeping a check on ammonia is vital to providing the best thriving environment for all organisms in the aquaponic system.
Why is it necessary to convert Ammonia into Nitrates?
- Ammonia is too harmful to fish to the extent that it will soon lead to their death unless converted into a less toxic nitrogen form (nitrates) or diluted to a non-toxic level. It also leads to nitrite poisoning.
- Plants absorb the nitrogen in the form of nitrates. The plants cannot readily absorb the ammonia. So plants will not get many nutrients out of high levels of ammonia.
Effect of Environment on Ammonia
The rise in temperature and pH levels make the ammonia even more toxic. The following test checks the total concentration of ammonia but there are actually two kinds of compounds included in the results with one being more harmful than the other. The levels of pH and temperature will determine the concentration of each ammonia type in water.
Moreover, ammonia keeps changing to ammonium (NH4+) and vice versa, with the relative amount of each depending on the water’s pH and temperature.
Ammonium is comparatively harmless to fish as compared to Ammonia. At higher temperatures and pH, more of the nitrogen is in the toxic its form.
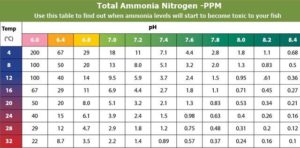
Temperature
From the above chart, you can determine the ammonia levels that are tolerable in your aquarium before it harms the fish. As you can see even small concentration of ammonia can be harmful to your fish at high water temperature. On other hands, the fish is able to tolerate higher ammonia levels at lower temperature. Check this post about optimum temperature range in aquaponics here.
This is also valid for DO (dissolved oxygen). The cold water has the ability to store more dissolved oxygen than the equal volume of warm water. Something we should all know! Check more about the role of dissolved oxygen in aquaponics here.
Overstocking your tank with fish is fraught with danger if any of these parameters are pushed beyond their tolerable limits. Check the fish to water ratio here.
You will get more control over your system health by learning about the relationship between water temperature and ammonia. This way you will be able to identify and mitigate the risk in the case of temperature spikes in summer and stressed fish.
Also, read: What are the Water Parameters that affect Aquaponics Cycle
The relation between pH and Ammonia
Ammonia is directly proportional to the pH
- The higher pH levels lead to the higher the ammonia levels and eventually the lower ammonium level.
- The lower levels of pH results in lower ammonia levels in a system and eventually higher ammonium level.
You should aim for a low pH level in your system to make ammonium the dominant element in your system.
Many people want to maximize the efficiency of Nitrification. A higher pH level enhances the oxidation efficiency in a new system that makes the people lean toward having higher pH in the system. However, as the system gets mature it adapts, and you can achieve the efficiency at lower pH values.
We suggest a pH level of 6-6.4.
How to Test Ammonia?
 You can test Ammonia levels by test strips, which is available at any local pet stores, or you can purchase online. Check the prices online here.
You can test Ammonia levels by test strips, which is available at any local pet stores, or you can purchase online. Check the prices online here.
Add a sample of the tank water in a clean container, take a test strip and dip it in the water for about 10 seconds.
Take the strip out, shake out any excess water, and immediately compare the color to the comparison chart that usually comes with the test strips.
How to reduce ammonia levels
You can bring down ammonia in aquaponics by increasing the conversion or reducing the source:
1. Increase nitrification efficiency
This method increases the oxidation rate of the ammonia or ammonium, expediting the conversion into nitrates, which plants can take up. Try to have non-toxic nitrates in your system.
2. Reduce the quantity of nitrogen going into your system
Overfeeding cause ammonia levels to spike up. To avoid this, cut back on feeding those fish. Dead fish secrete ammonia as it decomposes. Remove it If you find any. Stop feeding that tank for a day. The rotting feed also triggers the same effect as a dead fish.
Read in details about the prevention and treatment of nitrite poisoning here.